 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
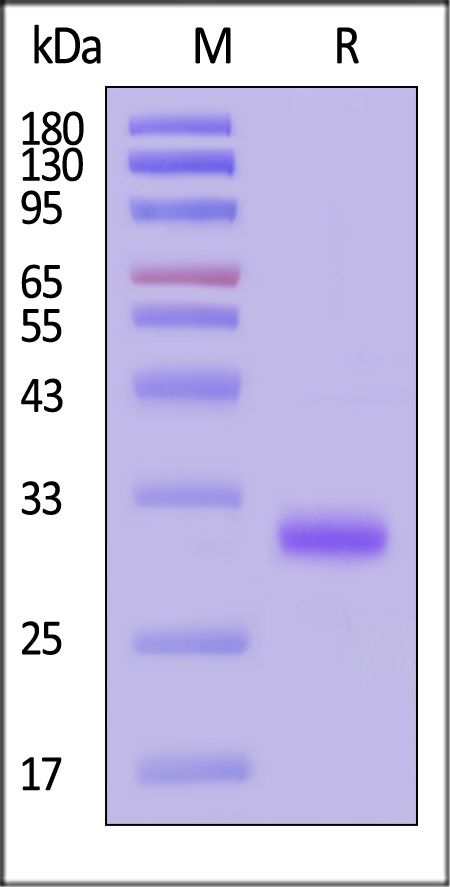
| IG3-M5214 | Mouse | Mouse IgG3 Fc Protein, Tag Free (MALS verified) |  |
|
|
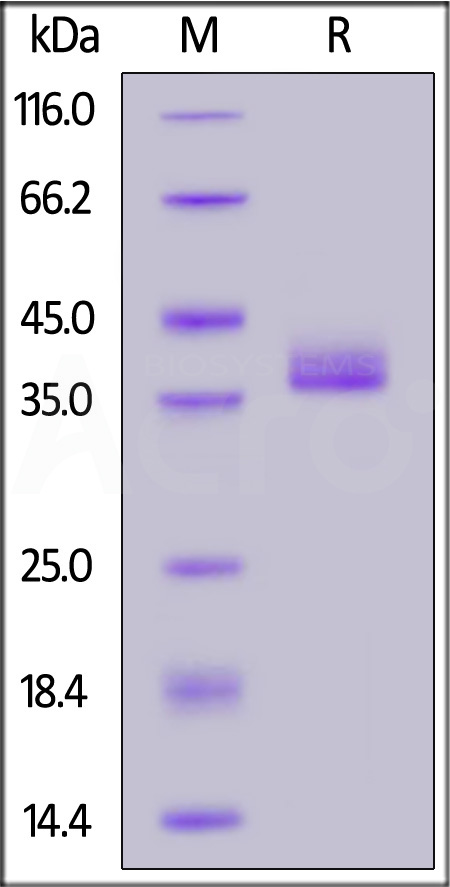
| IG3-H5200 | Human | Human IgG3 Fc Protein, Tag Free (MALS & SPR verified) |  |
|
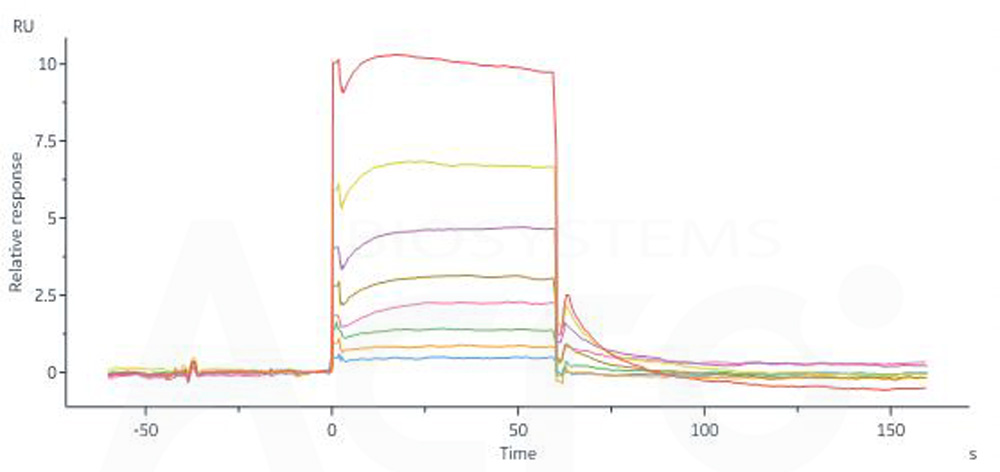
Human FCGRT&B2M Heterodimer Protein, His Tag (Cat. No. FCN-H52W7) captured on CM5 Chip via anti-His antibody can bind Human IgG3 Fc, Tag Free (Cat. No. IG3-H5200) with an affinity constant of 2.82 μM as determined in SPR assay (Biacore 8K) (QC tested).
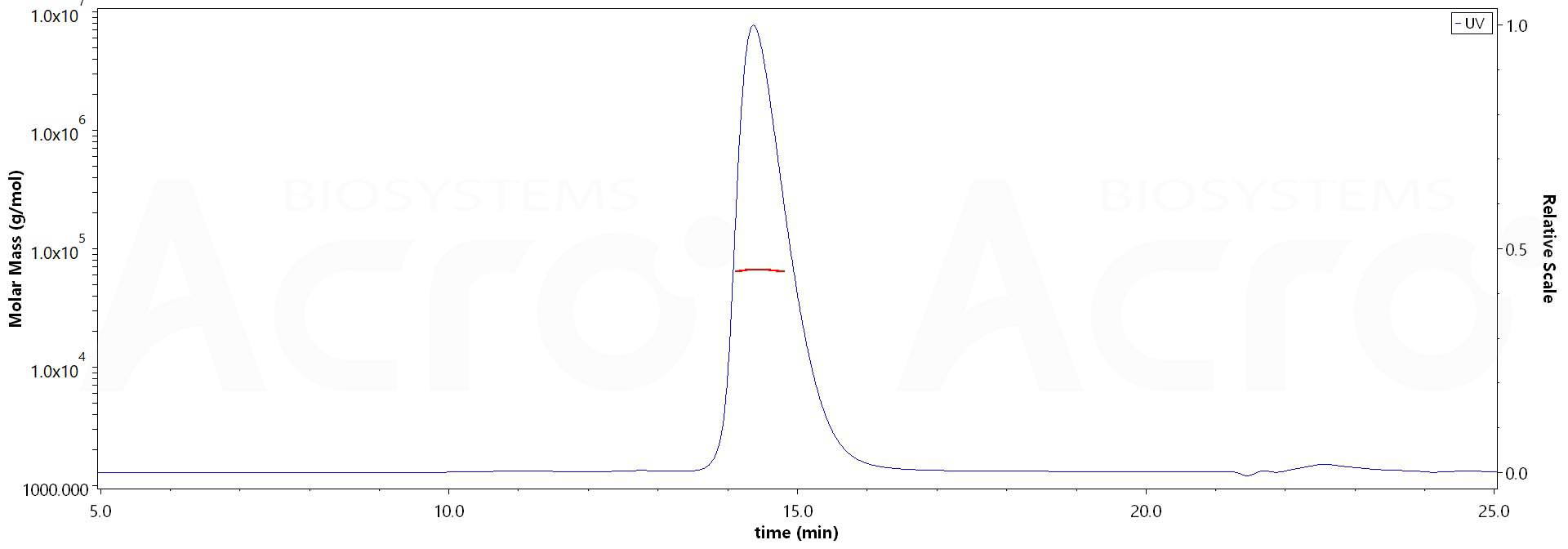
The purity of Mouse IgG3 Fc Protein, Tag Free (Cat. No. IG3-M5214) is more than 90% and the molecular weight of this protein is around 60-70 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Imlifidase | Mac-1 | Approved | Hansamed Inc | Idefirix | EU | Rejection of renal transplantation | Hansa Biopharma Ab | 2020-08-25 | Rejection of renal transplantation; Kidney Diseases; Purpura, Thrombotic Thrombocytopenic; Rejection of organ transplantation; Renal Insufficiency, Chronic; Guillain-Barre Syndrome; Kidney Failure, Chronic | Details |
| Imlifidase | Mac-1 | Approved | Hansamed Inc | Idefirix | EU | Rejection of renal transplantation | Hansa Biopharma Ab | 2020-08-25 | Rejection of renal transplantation; Kidney Diseases; Purpura, Thrombotic Thrombocytopenic; Rejection of organ transplantation; Renal Insufficiency, Chronic; Guillain-Barre Syndrome; Kidney Failure, Chronic | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| AT-02 | AT-02 | Phase 2 Clinical | Attralus Inc | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details | |
| AT-02 | AT-02 | Phase 2 Clinical | Attralus Inc | Immunoglobulin Light-chain Amyloidosis; Amyloidosis | Details |
| Anti-CD33/anti-FcgammaRI bispecific antibody (UCSD) | Phase 1 Clinical | University Of California San Diego | Leukemia, Myeloid, Acute | Details |
This web search service is supported by Google Inc.
